
Pennyroyal (Mentha pulegium) By Valter Jacinto | Portugal Creative Commons License Some Rights Reserved
Pennyroyal is traditionally used to induce sweating in order to treat colds and to induce menstruation and to induce abortion. These effects are probably caused by the patient' body trying to rid itself of the pennyroyal which is poisonous. Pennyroyal contains a chemical that causes liver damage.
 Pennyroyal (Mentha pulegium) By Valter Jacinto | Portugal Creative Commons License Some Rights Reserved |
Pennyroyal is classified as a diaphoretic (causes sweating) and as a emmenagogue (induces menstruation). It has traditionally been used to treat headaches and colds. It is eaten or drunk as a tea made from leaves or the essential oil distilled from the leaves and flowers.[5]It has been used to induce abortion.[5] It is sometimes used as an to get rid of insects.[6] It is comtimes used in pet shampoos as an anti -flea agent, but this is considered dangerous because of the toxic effect it can have on the pet.[4] The essential oil of pennyroyal is used in aromatherapy. [7]
None of the traditional uses are recommended by medical authorities. It's effectiveness for the uses it's traditionally used for have not been scientifically proven and pennyroyal is highly toxic to the liver.[1] [2] [8] Deaths have been documented due to drinking mint tea with oil of pennyroyal in it.3 When a person dies of pennyroyal poisoning, a minty smell can be detected on their breath.[2]
It is not proven which ingredients are responsible pennyroyal's supposed medical uses, as none of those uses have been proven. The main chemical found in the leaves and flowers is pulegone,[5] which is the agent that causes pennyroyal's toxicity. Pulegone (IUPAC name:5-methyl-2-propan-2-ylidenecyclohexan-1-one)[9], is metabolized to methofuran when ingested. In smaller amounts Methofuran causes neurological effects such as dizziness, seizures, and unconsciousness. In higher amounts it causes liver failure.[1] [8]
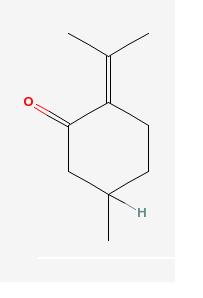 Pulegone |
Pulegone is a monoterpene ketone[12] that can be identified by its characteristic H-NMR spectrum:H-NMR: d 1.02 (3H, CH3), 1.79 and 1.99 (6H), C=C(CH3)2, and C-NMR spectrum:22.1, 22.5, 23.4 (3xq, 3CH3), 28.9, 33.2, 51.2 (3xt, 3CH2; DEPT-135, all three ‘down’ peaks), 31.9 (d, CH), 132.2, 142.3 (2xs C=C), 204.5 (s, C=O)].[13] It can be isolated by fractional distillation.[13]
An account of its synthesis could not be found as of this writing.
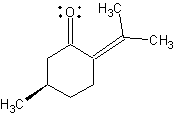 Pulegone |
|
Pulegone Ball and Stick Structure |
Bibliography
[1] Pennyroyal. Beth Israel Deaconess Medical Center. Retreived Oct. 7, 2008 http://www.bidmc.harvard.edu/YourHealth/HolisticHealth/HerbsandSupplements.aspx?
ChunkID=111712
[2] American pennyroyal (Hedeoma pulegioides L.), European pennyroyal (Mentha
pulegium L.). Natural Standard (www.naturalstandard.com). Retreived Oct. 7, 2008. http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-
pennyroyal.html
[3] Multiple Organ Failure After Ingestion of Pennyroyal Oil From Herbal Tea in Two
Infants. American Academy of Pediatrics. Retreived Oct. 7, 2008. http://pediatrics.aappu
blications.org/cgi/content/abstract/98/5/944
[4] Reduced Chemical Management of Fleas. P. G. Koehler. Retreived Oct. 7, 2008. http://edis.ifas.ufl.edu/IG106
[5] PENNYROYAL. Simon, J.E., A.F. Chadwick and L.E. Craker. Retreived Oct. 7, 2008.
http://www.hort.purdue.edu/newcrop/med-
aro/factsheets/pennyroyal.html
[6] 2.16 Pennyroyal. Rene Burrough. Retreived Oct. 7, 2008. http://www.henriettesherbal.com/faqs/medi-2-16-pennyroyal.html
[7] Essential Oils A-Z. Aromatherapypoint.com. Retreived Oct. 7, 2008.
http://www.aromatherapypoint.com/essentialoils/
[8] Pennyroyal. Memorial Sloan-Kettering Cancer Center. Retreived Oct. 7, 2008. http://www.mskcc.org/mskcc/html/69328.cfm
[9] Pulegone - Compound Summary. PubChem. Retreived Oct. 7, 2008. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6988&loc=ec_rcs
[10] Mentha pulegium. Botanical Society of the British Isles. Retreived Oct. 7,
2008. http://www.bsbi.org.uk/html/mentha_pulegiu
m.html
[11] Hedeoma pulegioides (L.) Pers.American false pennyroyal. USDA Natural
Resources Conservation Service. Retreived Oct. 7, 2008. http://plants.usda.gov/java/profile?
symbol=HEPU
[12] Chen LJ, Lebetkin EH, Burka LT. Drug Metab Dispos., 2001,
29, 1567 "Metabolism of (R)-(+)-pulegone in F344 rats" PMID: 11717176 [PubMed - indexed for MEDLINE]
[13] V. K. Agnihotri , S. G. Agarwal , P. L. Dhar, R. K. Thappa , Baleshwar , B. K.
Kapahi , R. K. Saxena , G. N. Qazi . Flavour and Fragrance Journal, 2005
20, 607, "Essential oil composition of Mentha pulegium L. growing wild in the north-
western Himalayas India" http://mutex.gmu.edu:2155/cgi-
bin/fulltext/110503024/PDFSTART DOI: 10.1002/ffj.1497
Search Log