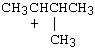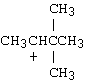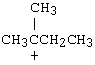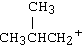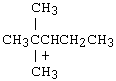Questions for Chapter 8[Answers below] |
1. |
2-Hexyne will not react with: |
A) H2, Pt B) Br2 |
|
C) NH3 D) H2SO4 |
|
E) KMnO4/H2O
|
2. |
Which reaction of an alkene proceeds with anti addition? |
A) Hydroboration/oxidation B) Bromination |
|
C) Permanganate oxidation D) Hydrogenation |
|
E) Epoxidation
|
3. |
Addition of 2 mol of HCl to 1-butyne would yield: |
A) CH3CH2CH2CHCl2 B) CH3CH2CCl2CH3 |
|
C) CH3CH2CHClCH2Cl D) CH3CH2CH=CHCl |
|
E) CH3CHClCHClCH3
|
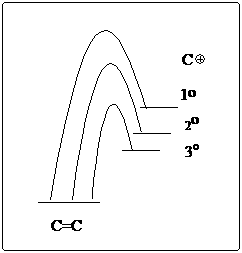
4. |
Which of the following carbocations would NOT be likely to undergo rearrangement? |
A) |
|
B) |
|
C) |
|
D) |
|
E) |
|
5. |
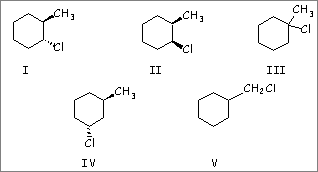
Treating 1-methylcyclohexene with HCl would yield primarily which of these? |
| A) I |
|
| B) II | |
| C) III | |
| D) IV |
|
| E) V |
6. Arrange the following set of compounds in order of rate of addition of HCl. [Answer]
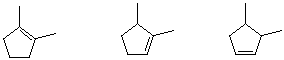
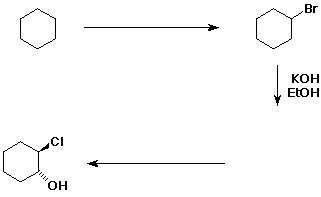
7. Complete the following synthetic sequence by adding the missing reagents or products. [Answer]
8. a) Complete each of the following reactions by drawing the structure of the expected final major product. Show the stereochemistry if the reaction is stereospecific. [Answers]
[Note: H2SO4 is a catalyst in this reaction.]
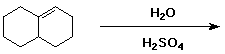
![]()
[Note: THF is the solvent in the first step of this reaction. B2H6 is equivalent to 2 BH3]
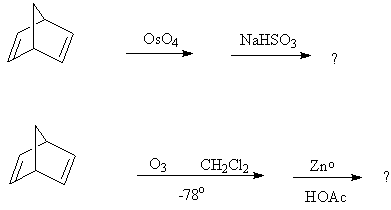
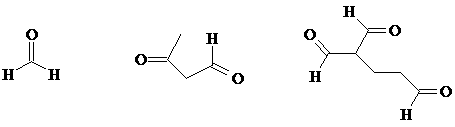
b) What is/are the oxidation product(s) from the following reactions? (There is excess oxidizing agent in both cases.)
c) A question in the Supplement asked you to draw the oxidation products for this reaction:
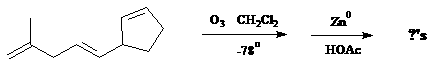
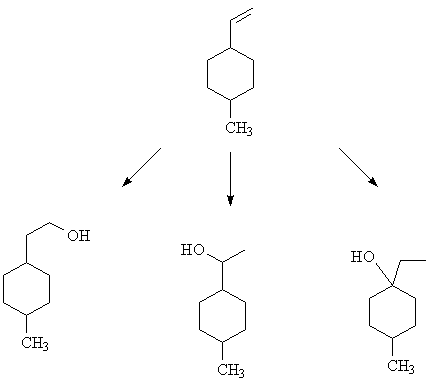
9. Overall hydration of the cyclohexane derivative shown below can be accomplished in three ways. What reagents/conditions were used to produce each of the products shown below? [Answer]
10. A Practice Problem in the Supplement asked: Draw the rate diagram that will account for the differences in the alkene relative rates of reaction as shown in Table II on an earlier page. Be sure to explain why the mono- and di-subtituted alkenes have similar rates and why the tri- and tetrasubstituted alkenes have similar rates. Table II showed this:
Effect of Methyl Substitution on the Relative Rate of Hydration of Alkenes, H2O, 25oC.
ethylene (6 · 10^−7); propene (1); cis-2-butene (1.7); 2-methyl-2-butene (4000); 2,3-dimethyl-2-butene (7000) [Answer]
For some Chapter 8 problems: see the MIT Open CourseWare site: Problem Set 3 (8a-d; 10a, b;); Problem Set 4 (1 (except CH2I2/Zn; CHBr3/KOH); 2 (except mCPBA); 3b; 4; 5c,e,f; 6).
1. C
2. B
3. B
4. C
5. C
6. a) middle - trisubstituted double bond;
b) left - tetrasubstituted double bond;
c) left - has most electron-donating alkyl groups that stabilize carbocation intermediate formation. Note that just because an alkene is more stable (b), doesn't mean that it is not the most reactive (c).
7. cyclohexane + Br2/CCl2 (heat or light) --> bromocyclohexane + HBr (or could use NBS instead of Br2).
Bromocyclohexane could undergo SN2 and/or E2 reaction with KOH/EtOH. Write both of these possible products for further consideration.
The last product is a chlorohydrin which is most easily obtained by addition of Cl2/H2O to an alkene. So the preceding reaction must be the elimination reaction (E2) to give cyclohexene. (The SN2 substitution reaction is a dead end.)

Working backwards, how could the alcohol product be
synthesized using an alkene that
is different from the one shown in the question?
8. b)
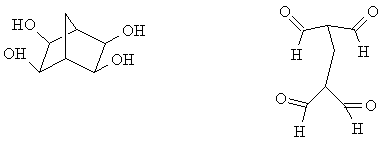
8. c)
9. The methods are listed below, in order. Look up the reagents and conditions in the text.
Hydroboration/oxidation; Oxymercuration/demercuration; Hydration
10. The first step in the hydration reaction is attack by the pi electrons on the reagent H3O+. The protonation occurs to give the more stable carbocation. Ethylene gives only a primary C+; propene and cis-2-butene both give a secondary C+ (of about the same energy); 2-methyl-2-butene and 2,3-dimethyl-2-butene both give a tertiary C+ (of about the same energy). So the two alkenes that both form a secondary C+ react at about the same rate (and faster than the alkene that forms only a primary C+). The two alkenes that form a tertiary C+ react at about the same rate (and faster than the alkenes that form a secondary C+). In the rate diagram below, only the C+ categories are shown, and not the individual reacting alkenes.