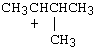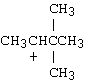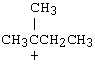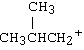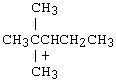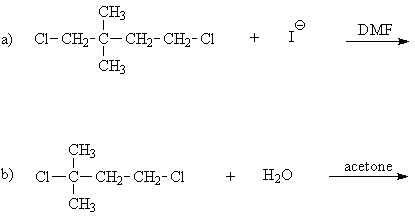Questions for Chapter 7[Answers 1.-6. below] |
1. |
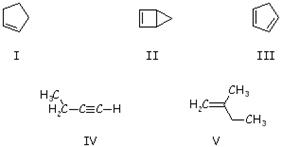
2-Pentyne is a constitutional isomer of which of these?
|
A) I B) II |
|
C) III D) IV |
|
E) V |
2. |
Dehydration of alcohols requires a ___________ catalyst.
|
3. |
In a dehydration reaction, the leaving group is _________.
|
4. |
Which of the following carbocations would NOT be likely to undergo rearrangement?
|
A) |
|
B) |
|
C) |
|
D) |
|
E) |
|
5. Arrange the following sets of compounds in order with respect to their stability. Write "MOST" and "LEAST" under the compounds with the highest and lowest stabilities in each set.
a) stability order
![]()
b) stability order
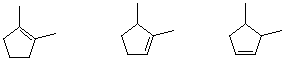
6. Which mechanism, E1 or E2, will occur in each reaction. Explain your answer. (Draw the reacting structures before attempting to answer.)
a) t-butyl chloride and sodium methoxide b) 1-tert-butyl-1-chlorocyclohexane and methanol
7. One of these bicyclic molecules undergoes E2 elimination with NaOCH2CH3/CH3CH2OH faster than the other. Which one reacts faster? Explain.
To begin solving this problem, you need to take the planar bicyclic system (a trans-decalin) and show it in the proper chair conformation. A trans-decalin template is provided - put in the two methyl groups trans to each other at the ring-junction carbons and then draw in the bromine atom with the proper stereochemistry. Proceed with the analysis as you would with the monocyclic cyclohexane.

[Answer]
8. The mechanism for the formation of trans-alkenes from a disubstituted alkyne and an active Group I metal involves a radical anion intermediate.
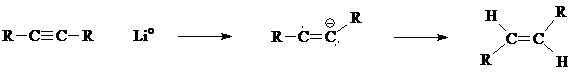
What is the hybridization and geometry at each of the two carbon atoms in the intermediate?
Draw a molecular orbital picture of the radical anion intermediate.
[Answer]
9. Explain how you arrive at the answer to the following problem. [Answer]
Competition experiments are those in which two reactants at the same concentration (or one reactant with two reactive sites) compete for a reagent. Predict the major product resulting from each of the following competition experiments:
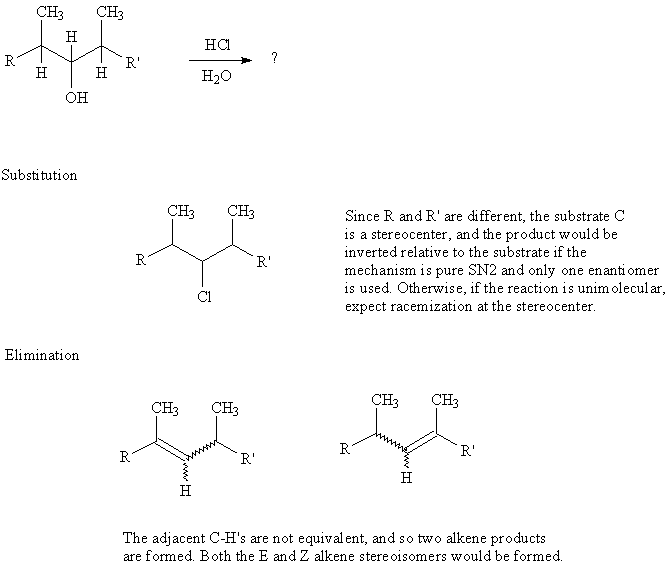
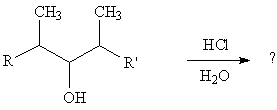
10. Write all possible products resulting from treatment of the alcohol shown with HCl/H2O. (Start with an acid/base reaction between the hydrochloric acid and hydroxyl group of the alcohol.) [Answer]
For some Chapter 7 problems, see the MIT Open CourseWare site: Problem Set 3 (2; 3a, b); Problem Set 5 (3).
1. A
2.acid (H+)
3. H2O
4.C
5. a) middle - trisubstituted double bond;
b) left - tetrasubstituted double bond;
6. a) E2 A strong negatively charged base is present, so bimolecular. With a tertiary substrate there will be no SN2, so the mechanism is E2.
b) There is no strong base, and methanol is an ionizing solvent. The substrate is tertiary. So E1.

In the structure on the left, the Br must be axial because it is trans to the methyl at the nearest bridgehead (one up, the other down). The bridgehead carbon has no H's that can be eliminated. The other beta-carbon has two H's. One of them is anti to the Br (shown in red) and so eliminates readily.
In the structure on the right, the Br must be equatorial because it is cis to the methyl at the nearest bridgehead (both down). The bridgehead carbon has no H's that can be eliminated. The other beta-carbon has two H's. Both of them are gauche to the Br; there is no H that is anti and so the elimination is slower.
8. The radical carbon has 2 sigma bonds and so is sp-hybridized (the lone electron does not count toward determining hybridization) with a linear geometry (as shown in the picture below).
The anionic carbon has 2 sigma bonds + 1 pr. nb electrons and so is sp2-hybridized with trigonal planar geometry.
The radical carbon uses its 2 sigma bonds to bond to an R group and to the adjacent carbon. There are two unhybridized p electrons: one of these is in a p orbital that is part of the pi bond to the adjacent carbon and the other p electron is the lone radical electron.
Of the three sp2 orbitals on the anionic carbon, two of them form sigma bonds - one to the R group and one to the adjacent carbon. The third sp2 orbital contains the non-bonded electron pair. The anionic carbon uses the unhybridized p orbital to contain the fifth electron that is part of the pi bond.
The p orbital shown in pink contains the lone electron and is mutually perpendicular to the sigma-framework and the p orbitals of the pi bond. The pi bond is the overlap of the two electrons in the p orbitals shown in blue.
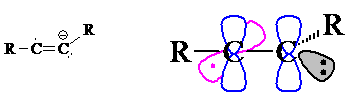
9.a) There are two potential chloride leaving groups, both on primary C's. Thus, a cationic mechanism is not likely. Further, the DMF solvent is polar aprotic, which, although polar, is not a good ionizing solvent. The iodide is a better nucleophile than it is a base, and so the reaction is most likely substitution, rather than elimination, to give an R-I product. Of the two primary C-I sites, the one with beta branching is less favorable (because of greater steric hindrance) than the one without branching near the reaction site.
.b) There are two potential chloride leaving groups, one primary and the other tertiary. The reactant is water, which is probably present in excess, as a co-solvent with acetone (a non-nucleophile, non-base). These are not bimolecular conditions. Water is a fairly good nucleophile, but a weak base, so substitution is probably favored, as usual, in a unimolecular reaction. For a unimolecular substitution, ionization will take place at the tertiary C-Cl bond and, after attack by water, and deprotonation of the oxonium ion intermediate, will give the tertiary alcohol as the product.
10. Protonation of the OH group by HCl gives the oxonium ion, R-OH2+. The leaving group is charged in the substrate. When it leaves, it leaves as the neutral H2O, which is a very good leaving group.