Questions on Alcohols and Ethers
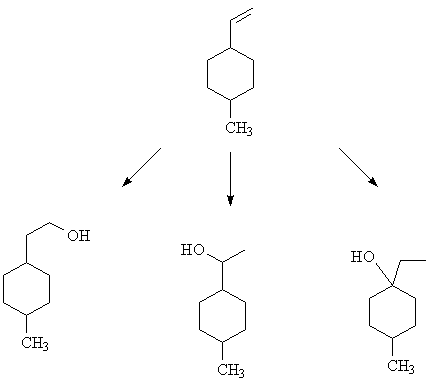
1. Overall hydration of the cyclohexane derivative shown below can be accomplished in three ways. What reagents/conditions were used to produce each of the products shown below? [Answer] [Also in Chapter 8 questions.]
2. a) Name the first compound according to the IUPAC system and the second compound with a common name.
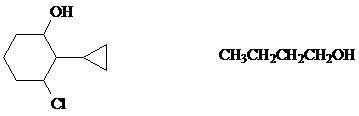
c) Explain why propanol boils at a higher temperature than the corresponding hydrocarbon.
Explain why propanol is soluble in water but hexanol is not soluble in water.
d) Why does the reaction of (CH3)3CCl with OH- give no (CH3)3CCl? There is a product from the reaction - what is it?
e) There are two different reactions you could use to synthesize n-butyl bromide from 1-butanol - what are they?
[Answers]
3. For some Chapter 11 problems, see the MIT Open CourseWare site: Problem Set 4 (1 [except CH2I2/Zn; CHBr3/KOH]; 3d,f,g; 5a [note oxidizing conditions],d); Problem Set 8 (1-3, 4c)
1. The methods are listed below, in order. Look up the reagents and conditions in the text.
Hydroboration/oxidation; Oxymercuration/demercuration; Hydration
The hydroxyl group takes precedence to determine the parent name; it is substituted on the cyclohexane ring. The other groups are substituents and named in alphabetical order.
n-butyl alcohol
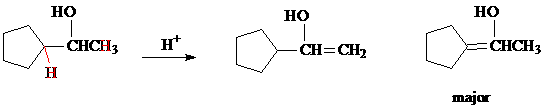
b) There are two beta-H's that can be eliminated with the OH group, shown in red. The two products are drawn below. The major product is the more stable and more highly substituted alkene.
c) Propanol can hydrogen-bond to the solvent water molecules. The non-polar hydrocarbon cannot hydrogen-bond and so is insoluble.
The hexanol has a larger non-polar hydrocarbon area and so resembles a hydrocarbon, even though the OH of hexanol is capable of hydrogen-bonding. Overall, the non-polar character outweighs the polar character.
d) This is tert-butyl chloride, a tertiary halide, which will not undergo a substitution reaction. With the strong hydroxide base, the E2 elimination reaction takes place to give 2-methylpropene.
e) CH3CH2CH2CH2OH ---> CH3CH2CH2CH2Br
-This is a substitution reaction. OH is a poor leaving group and cannot be directly substituted by Br-. Use HBr to first protonate the OH group and turn it into the good leaving group OH2. Br- can then displace the good leaving group in an SN2 reaction (primary substrate).
-A very good method for converting primary alcohols to bromides is to use PBr3. The reaction mechanism is a variation on converting a poor OH leaving group into a good leaving group (you can see the mechanism in the textbook).