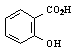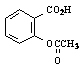Report Form:
The Reaction Classification is the mechanistic classification for the reaction (e.g. "Unimolecular Elimination (E1)", not "Synthesis
of Cyclohexene"). Write the complete mechanism for the reaction on the back of the report form. Include arrows, non-bonded electrons,
formal charges, resonance contributing structures, etc. as necessary. See your lecture textbook for correct mechanisms.
Calculations:
The textbook includes a brief discussion of calculations that are relevant for synthesis experiments. Please read it.
Your calculations should appear on a separate page. The calculations should be done in your notebook on pages that are a continuation of the experiment in your notebook (and so properly titled and dated). Turn in the copy as part of your report and keep the original in your notebook. Clearly label
all calculations and carry units (g, mol, etc.) throughout a calculation.
Include a calculation for determining the limiting reagent. Pay attention
to significant figures.
The "Calculation
of Theoretical Yield" table on the report form should be filled
out as illustrated below. Notice you do not enter the actual yield in this table; there is a space for that on the Report Form. Use abbreviated structural formulas, not molecular formulas, for all organic compounds.
Calculation
of Theoretical Yield:
The superscript
numbers in parentheses in the table above are the reference citation numbers for the literature
values for M.W. (and other properties) of the substances. Make sure each different substance has a unique citation number with the corresponding number in the Citation section of the report form. If you cite the same substance in different parts of the report form (e.g. its M.W. in the table and then its boiling point), use the originally-assigned reference citation number.
Do not add
atomic weights to obtain molecular weights. Be sure the source you use for M.W. reports at least 4 significant figures.
* 5.0 mL of liquid acetic anhydride was used in the example reaction
above. The mass that is reported in the table was calculated from
the volume used in the experiment and literature density. Acetic anhydride
was present in excess: 0.053 mol / 0.0148 mol = 3.6:1 mole ratio (and
thus the justification for measuring its volume, not mass, to only
two significant figures). The calculation should be shown on the separate
calculation page, not on this summary of results form.
Salicylic acid
is calculated to be the limiting reagent in the synthesis.
Note that the moles of products theoretically produced are the same
as the moles of the limiting agent used.
Click
here to see an explanation of the calculations for syntheses
where a strong mineral acid, such as HCl or H2SO4, is used.
Citations:
Citations must conform to the usual style and permitted sources. Properties
such as molecular weights, m.pt.'s, etc. should be sequentially numbered
on the report form with corresponding numbers in the Citations section
at the bottom of the Report Form. If both the molecular weight and
m.pt., for example, come from the identical source, then both properties
should be assigned the same reference number and cited only once. Also see the lab Manual.
Attachments:
A Separation Scheme (unless it has already been submitted); experimental
IR spectrum of product (if taken); and literature spectrum (IR, NMR [Chem 318])
of product for comparison (regardless of whether an experimental spectrum
was obtained) with the relevant absorption
peaks labeled. Be sure to cite the source(s) for your literature spectrum.
Assemble the
report in this order:
Report form (with mechanism on back)
Calculation page(s)
Spectra (exp'tl, lit. with mark-up)
Separation Scheme
(see Manual for an example)
Submit the product
in properly labeled vial
There is no written
analysis report. |