A. Fill in the Report Form.
B. Attach a photocopy of your experiment notebook page(s) that show your drawing of your developed TLC plate(s).
C. If your Rf calculations are not on the photocopy, then either photocopy the calculations, or include a new calculation page that shows how you obtained the Rf values shown on the Report Form. Attach the calculations to the Report Form.
D. Written Report as explained below (should be typewritten, 12-pt. font, 1-inch margins, printed on one side; structures should be hand-drawn).
Before writing your report, In your organic chemistry textbook, please review these topics:
· resonance structures, especially for carbonyl (C=O) compounds;
· polar/non-polar functional groups, dipole arrows, and determining molecular polarity;
· dipole-dipole interactions and hydrogen-bonding (a strong dipole-dipole interaction).
After visualizing your thin-layer chromatography results, no doubt you established the elution order of the three compounds as: fluorene > fluorenone > fluorenol. This might have been a surprise. After all, the stationary phase, silica, is quite polar and the order of polarity of the three compounds (as stated correctly in the experiment) is not the elution order.
However, after considering the structures of the fluorene derivatives and the structure of silica, you should be able to account for the elution order.
1. First, account for the order of polarity of the three compounds by considering parts a) and b), below.
a) Fluorene is essentially non-polar. Briefly explain, with respect to atoms that are present and absent in fluorene, and their relative electronegativities, why fluorene is non-polar.
b) The dipole moment of 2-propanol is 1.66D and that of acetone is 2.69D. Draw the structure for each of these, and then draw the minor, but important, resonance contributing structure for acetone. It is the resonance hybrid structure of ketones that accounts for their greater polarity compared to alcohols of similar structure.
c) Draw a resonance contributor and the resonance hybrid structure for fluorenone.

2. a) Although fluorenone is more polar than fluorenol, more specific interactions between fluorenol and silica account for its slow migration in thin-layer chromatography. Below is a picture of the partial structure of silica. Using abbreviated structures for fluorenol and fluorenone, show the specific intermolecular interactions between these compounds and silica. (Draw in extra silanol groups, as necessary. Use abbreviations such as R2CHOH and R2C=O. A good drawing will show at least two molecules of fluorenol (interacting in different ways with silica) and one molecule of fluorenone and will take into account the review topics, above.) Be sure to include all non-bonding electrons in your drawing.
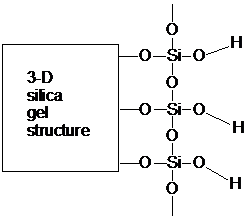
b) Briefly summarize the interactions between silica and the three compounds that accounts for the elution order in TLC.
Assemble the Report in the order A.-D., above.