
© 2006 Robert Shantz
| Table of
Contents |
 © 2006 Robert Shantz |
Heliotrope is a plant, also called turnsole and girasole. Its scientific name is Heliotropium arborescens of genus Heliotropium. Heliotrope produces clusters of sweet scented flowers in summer and is not tolerant of cold weather. The flower fragrance is the most noticeable (cherry pie) at sunrise and sunset and may be enhanced by giving plants some water. Its flowers color is purple (primarily), blue and white. The plant needs rich soil, lots of water and sun to partial shade. The plant grows to 1-2 feet tall. [3]
The plant occurs rather sparingly in the southeastern states from Florida up to New Jersey and occasionally into New England; Most problems have been reported in Australia, since they have more cattle.
Heliotrope generates by seed (annually) and cutting. The cuttings are better to be taken from especially fragrant plants between late spring and early summer. Seed propagation takes 3-4 weeks at temperature of 70 degrees Fahrenheit, and can be unreliable. Softwood cuttings will root in about two weeks at low temperature. The plant may be grown as plotted plant.[3]
Toxicity results from ingestion of a large quantity over an extended period of time. All parts of the heliotrope are poisonous and will cause gastric distress in humans and animals. Major species that are effected are Herbivores, cattle, horses, pigs, poultry, and sheep (which are less vulnerable). Heliotrope has slow acting liver toxin, which causes liver damage (atrophic hepatosis). Sometimes it has a high death rate in cattle; no known human losses have been reported in USA. In most cases of toxicity, animals consume large amounts of the heliotrope over an entire season without any developing signs. However underneath, the hepatocyte changes continue until the second season, when the affected animal will have serious poisoning.In addition, consumption of this plant may effect milk quality.
Toxicity Sighns
The toxin causes progressive changes in characteristics of liver atrophy, and might cause photosensitization. A sudden hemolytic jaundice as a response to the toxic, and eventually death.
Toxic principle
The poison of heliotrope is from two types of pyrrolizidine alkoilds
and their respective N-oxides. Also allylic esters of pyrrolizidine
alkaloids which are also hepatotoxic. The plant contains about 3%
alkaloids, mostly as N-oxides. [5] Two pyrrolizidine alkaloids, Indicine (1)
and lycopsamine (2), have been isolated from Heliotropium.[7] N-Oxide Indicine Lycopsamine The N-oxides of the alkaloids are more soluble in
water and therefore
can be pwdabsorbed rapidly in human body. In this process N-oxides stay
intact, whereas the parent alkaloids are significantly degraded in the
rumen, the first division of the stomach. Metabolism to the much more
toxic pyrrole compounds occurs in the tissues after the toxin
absorption.[1] Prevention can be done
partially by supplying the cattle with plenty cobalt to form vitamin B12.
This vitamin helps to decrease the effects of pyrrolizidine alkaloids.
Also cobalt can be used for detoxification before liver damage
happens. The Fragrant flowers are used in the
perfume production. There is a color "heliotrope" named after the plant
Heliotropium (Heliotrope), which is a moderate, light, or brilliant violet
or purple. [6] There is
a scientific instrument named after the plant,invented by physicist Carl
Friedrich Gauss. The word "Heliotrope" in detail means "the sun" and "to
turn". Therefore, it is a fitting name for the instrument which does just
that reflects the sun toward a given point.[2] The
Scifinder program was used in searching for more information on our plant
poisons. I began the search by using Locate Substances option from the
LOCATE window and entering the name of my first poison, N-oxide Indicine.
The new page showed three structures of the compound that looked the same,
and all were called N-oxide indicine. But only the third option
(#41708-76-3) took me to the references (58) I was looking for. It gave 83
results, but after removing the duplicates it was 79 results. Then I
refined them down to only journals as the document type preferred (now 60
results). Changing the language to only English gave me 56 results. At the
end, I refined my search by Research Topic, written synthesis. So I ended
up with 14 results. But unfortunately most of the options would not work
or were not really related. Also the Elsevier journals would only appear
as abstracts and I could not reach the full text. The same procedure was
done for Lycopsamine. The search gave only one structure, which showed 88
references without any duplicates. There were only 86 journals and 78 of
them were in English. And references were limited to 18 by Research Topic
of synthesis. Unfortunately, the same problems as before with n-oxide
Indicine was seen. The reference # 9 here, was repeated for both searches
and was a very good source of information on both compounds. 8.Powis, G., Ames, M.M., & Kovach J.S. Res. Commun. Chem. Pathol. Pharmacol., 1979, 24, 559-569."On Relationship of the reductive
metabolism of indicine N-oxide
to its antitumor activity".Retrieved
March 7,
2006, from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=451340&dopt=Citation
9.Zalkow, L. H., Glinski, J. A., Gelbaum, L. T.,
Fleischmann, T. J., McGowan, L. S., & Gordon, M. M. J. Med. Chem.,
1985,28, number 6. "On Synthesis of Pyrrolizidine Alkaloids Indicine,
Intermedine, Lycopsamine, and Analogues and Their N- Oxides.
Potential Antitumor Agents". Retrieved on March 28, 2006, from
http://pubs.acs.org/cgi-bin/archive.cgi/jmcmar/1985/28/i06/pdf/jm00383a001.pdf 10.Niwa, H.,
Ogawa, T., & Yamada, K. Tetrahedron Lett.,
1989, 30, 4985-4986. " On An Efficient Enantioselective Synthesis of (+)-Indicine N-oxide, an Antitumor Pyrrolizidine Alkaloid". Retrieved March 27, 2006, from
[Top of Page]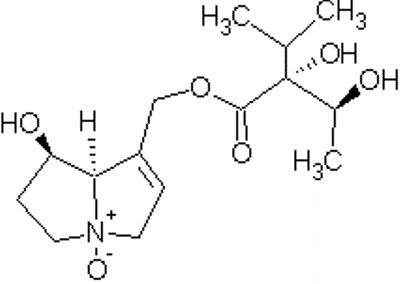 Drawn using ChemSketch
Drawn using ChemSketch 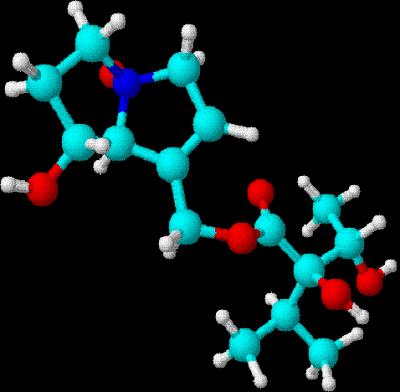 3D rendering using DSViewer Lite
3D rendering using DSViewer Lite
Indicine
N-oxide (from heliotrope) is an antitumor agent currently
investigated for treatment of brain tumors.[8]N-Indicine, initially showed promise as a
chemotherapeutic agent in cancer (solid tumours); prevoiusly it was found
useful in treating leukemia in infants, except that it produced liver
damage as a side effect in some cases. therefore, it was withdrawn from
further consideration.[5]Indicine and its N-oxide were first isolated
from Heliotropium indicumin 1961 in study of plants potential in
damaging the liver. N-oxide is a more active antitumor agent than
Indicine. Hydrolysis followed by oxidation produces N-oxide of the
Indicine.[9] It could
also be synthesized in its natural form in
five steps starting with lactone.[10] Drawn using ChemSketch
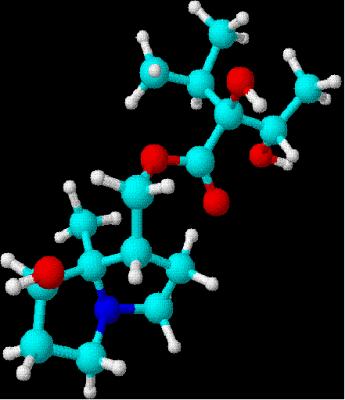 3D rendering using DSViewer
Lite
3D rendering using DSViewer
LiteIts an organic chemical and
stereoisomer to intermedine (both C15H25NO5).[7]Both of these streoisomers are infamous for
their tendency to not crystallize. Their seperation from mixtures has been
possible using high-performance liquid chromatography, ion-pair absorption
chromatography, and chromatography of their borate complexes.Lycopsamine occurs commonly in plants and causes
health hazards in herbal teas and in honey to human body.[9] Prevention and Treatment
Fun Facts
Searching for Chemical Information
Refrences
 1.Knight, A. P.; Walter, R. G. Plants Affecting the Skin and
Liver.International Veterinary Information Service: A Guide to Plant
Poisoning of Animals in North America. Retrieved Febuary 22, 2006, from http://www.ivis.org/special_books/Knight/chap4/chapter_frm.asp?LA=1
1.Knight, A. P.; Walter, R. G. Plants Affecting the Skin and
Liver.International Veterinary Information Service: A Guide to Plant
Poisoning of Animals in North America. Retrieved Febuary 22, 2006, from http://www.ivis.org/special_books/Knight/chap4/chapter_frm.asp?LA=1
2.Wikipedia The Free Encyclopedia: Kalmia latifolia.
Retrieved Febuary 22, 2006, from http://en.wikipedia.org/wiki/Heliotropium
3. Michigan Nursery and Landscape Association:Ornamental
Plants plus 3.0. Retrieved Febuary 24, 2006, from http://web1.msue.msu.edu/imp/modzz/00000709.html
4.Trinity College: Poisonous Plants. Retrieved
Febuary 24,
2006, from http://www.trinity.wa.edu.au/plduffyrc/subjects/science/useplants.htm
5.IPCS INTOX:International Programme On Chemical Safety.
Retrieved
Febuary 25,
2006, from http://www.intox.org/databank/documents/chemical/pyrralk/ehc080.htm
6.Psych Central: Dr. John Grohol .Retrieved
Febuary 23,
2006, from http://find.psychcentral.com/psypsych/Heliotrope
7.Reina, M., Gonzalez-Coloma, A., Gutierrez, C., Cabrera, R.,
Henriquez, J., & Villarroel, L. J. Nat. Prod., 1998,61,1418-1420." On Pyrrolizidine alkaloids
from Heliotropium megalanthum".
Retrieved Febuary 24, 2006, from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9834169&query_hl=11&itool=pubmed_docsum