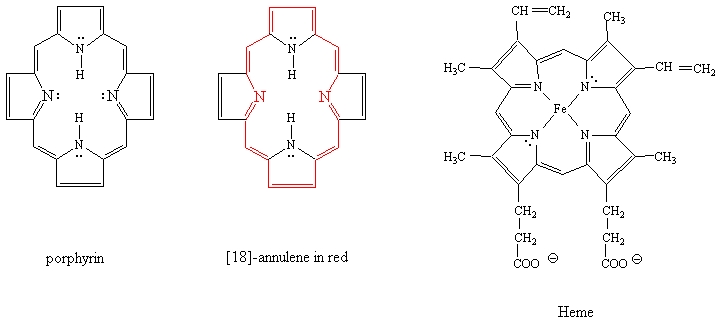
The histidine nitrogen that coordinates with the Fe(2+)
atom of heme is the one that is more basic, i.e. which does
not have its non-bonded electrons as part of the delocalized pi system.
The histidine that is close to the O2 coordinated to the Fe atom is
oriented with its partially positive hydrogen close to the electronegative
oxygen atom of O2.