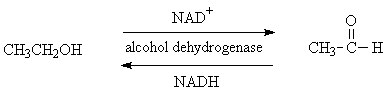
This view of alcohol dehydrogenase shows its secondary structure of beta-sheets and alpha-helices. The active site is barely visible.
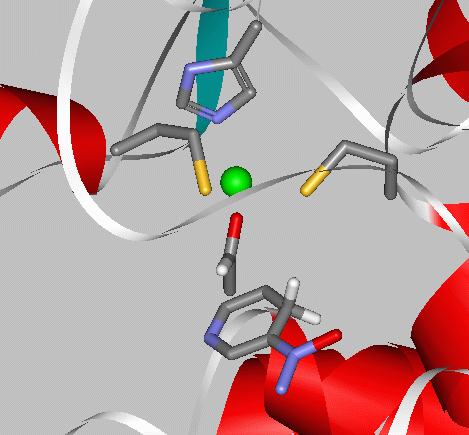
This close-up of the active site shows the Zn2+ cation coordinated with two cysteine anion residues (-CH2-S-), and a histidine residue (cyclic N-containing group). An acetaldehyde is coordinated to the zinc and the -OH group of a serine residue (not shown in the 3D picture). Held in position, the acetaldehyde is reduced to CH3CH2O- when a hydride (H:-) is released from the NADH. The reduction is completed when the oxygen is protonated by H+ that is ultimately released from the serine hydroxyl group (which in turn acquires a new proton by a series of relays of H+ from the enzyme surface).
Here is a Lewis-structure drawing of the molecules in the active site.
Toggle between the molecular view and Lewis structure of the active site
For your own copy of the original alcohol dehydrogenase PDB file to view later in DSViewerLite, right-click your mouse on the molecule pdb file link, then choose "Save link as..." Alcohol Dehydrogenase.pdb
Copyright 2020 S.W. Slayden all rights reserved