Questions for Chapter 6 (and related topics)
1. Hyperconjugation is best described as:
(A) delocalization of pi electrons into a nearby empty orbital
(B) delocalization of sigma electrons into a nearby empty orbital
(C) the effect of alkyl groups donating a small amount of electron density inductively into a carbocation carbon
(D) the shift of a carbon or hydrogen from one carbocation carbon to another
2. The following order of carbocation stability can be explained on the basis of

(A) hyperconjugation
(B) inductive effect
(C) both hyperconjugation and inductive effect
(D) neither hyperconjugation and inductive effect
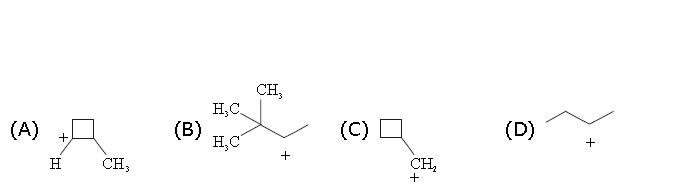
3. Which of the following is/are most likely to undergo a favorable hydride shift? [Hint: Draw in all hydrogen atoms.]
. 
4. [Hint: Draw in all hydrogen atoms.]

[Answers]
These are some simple questions concerning SN2 reactions:
1. Draw the product(s) of the SN2 reaction between (S)-2-bromooctane and sodium methoxide.
[Answer]
2. Put the following compounds in the order of their reactivity with azide anion:
5. Draw an energy diagram that shows the relative rates of reaction between bromide ion and ethyl-X where X is SH and OH.
[Answer]
These are some simple questions concerning SN1 reactions:
6. Draw the product(s) of the SN1 reaction between (S)-2-bromooctane and water.
[Answer]
7. Put the following compounds in the order of their reactivity in a solvolysis reaction with water:
n-hexyl bromide 2-bromo-2-methylpentane 3-bromohexane
[Answer]
8. Put the solvents in order of increasing rate of an SN1 reaction between tert-pentyl iodide and water.
acetone ethanol hexane
[Answer]
9. Which halide reacts fastest as a leaving group in an SN1 reaction?
10. Write a plausible rearrangement product for the ethanolysis reaction of 1-iodo-2-methylcyclopentane.
[Answer]
Try the problems from the MIT Open Course site Problem Set 6 (except 4b;, 9a,b; 10d, g; 11.) There are answers also.
Answers to hyperconjugation and rearrangement questions
1. (B) the sigma electrons of C-H (in our examples) are delocalized into an empty p orbital (in our examples). Delocalization by hyperconjugation is different from the inductive effect.
2. (C) Both hyperconjugation (delocalization of sigma electrons) and the inductive effect (polarization through the sigma bonds) explain the order of carbocation stability.
3.Both (A) and (C) result in the formation of a more stable tertiary carbocation.

4. (B) This carbocation can rearrange to an equally stable (identical) carbocation. There is no more stable possibility. All the other carbocations shown can rearrange to a more stable tertiary carbocation by a hydride shift.
Answers to Substitution questions
1. See the Solomons textbook section where the reaction between R-bromooctane and hydroxide are shown. Draw the mirror image of the substrate to make the S-bromooctane. Replace the hydrogen of OH- with CH3O- to make methoxide.
2. Draw the expanded structural formulas for all of these. If you can't figure out the structure, go to Chemspider.com or some other source. SN2 reactivity depends on steric accessibility to the attacking nucleophile, so generally Me > prim. > sec. > tert., vinyl, aryl. Remember, tertiary RL's don't react by SN2 mechanism. It makes no difference what the nucleophile is.
n-pentyl chloride (unbranched prim.) > 3-chloropentane (sec.) > neopentyl chloride (highly branched prim.) > 2-chloro-2-methylbutane (tert.)
3. The nucleophiles have a common nucleophilic atom. Regardless of the solvent, the more basic the nucleophile, the more reactive it is. The basicity/nuclophilicity order is:
sodium ethoxide > sodium hydroxide > sodium acetate
The base strength is the opposite of the strength of the conjugate acid. You should be able to draw the structures for these nucleophile/bases.
4. The reactivity of nucleophiles in the same Group depends on the solvent. The solvent is acetone, a polar aprotic solvent. It doesn't solvate anions very well. The best nucleophile will be the smallest atom with the highest concentration of negative charge.
F- > Cl- > Br-
5. X is the leaving group. Leaving group ability is correlated with basicity: the more stable (weaker) the base, the better the leaving group. Acidity increases down a column; conjugate basicity is the opposite. So HS- is a better leaving group than HO-.
The better the leaving group, the faster the reaction. The faster the reaction, the smaller the Ea ( energy of activation/energy hill).

6. The product is the racemized 2-octanol. See Solomons where the products are the (S)- shown and its mirror image, the (R)-, in approximately a 50:50 ratio.
7. (Draw out the structures before trying to answer the question.) 2-bromo-2-methylpentane is tertiary and so will be most reactive in an SN1 solvolysis reaction because it forms the most stable carbocation intermediate.
8. SN1 reaction rates are increased in an ionizing solvent. Ethanol is more ionizing than acetone. Hexane (a hydrocarbon) is non-polar and not ionizing.
9. Leaving group ability is correlated with basicity: the more stable (weaker) the base, the better the leaving group. Acidity increases down a column; conj. basicity is the opposite. So Br- is the best leaving group.
10.





