Questions for Chapter 3
1. Write a chemical reaction for an intramolecular acid-base reaction of neurontin. "Intramolecular" means the reaction takes place within just the one molecule shown.

[Answer 1]
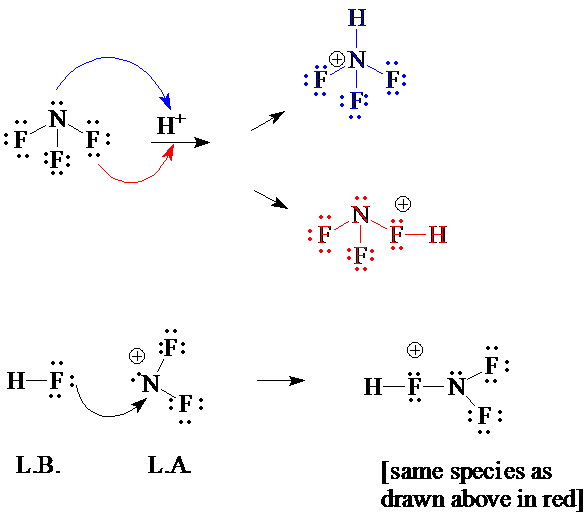
2. a) NF3 in the gas phase can be considered a bifunctional Lewis base, that is, its reaction with H+ can occur at the non-bonded electrons either on N or on F. Draw a complete electron-dot structure for NF3 . Show the two products of its reaction with H+. Remember to include electron-movement arrows.
b) The (NF3H)+ ion formed in one of the reactions above has a significantly long F2N−FH bond, suggesting that it may be perceived as an ion-dipole complex between the (NF2)+ cation and the HF molecule. Draw electron dot structures for these latter two species. Show how their Lewis acid-base combination results in the same product obtained from reaction between H+ and NF3 (reaction at F).
[Answer 2]
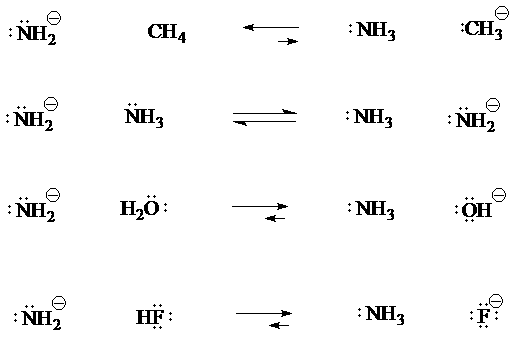
3. Write acid/base equations for the reactions of CH4, NH3, H2O, and HF with the amide anion base, (NH2)−. Which species are favored at equilibrium in each reaction, taking into account the acid/base strengths? [This question is in the Supplement.]
[Answer 3]
4. There is an olfactory sensor in mice that binds the volatile chemical (methylthio)methanethiol [MTMT]. This chemical is excreted by male mice to signal females that they are fertile. The receptor cannot be activated in the absence of copper ion (Cu2+). Experiments indicate that copper ion binds to MTMT before binding to the receptor. MTMT is H3CSCH2SH. (Note the presence of the thioether and the thiol functional groups.)
Draw an expanded structure for MTMT.
Assume that the MTMT and copper ion will engage in a Lewis acid-base reaction to form a complex.
Classify the two atoms in MTMT and the copper ion as either Lewis acids or Lewis bases. Show the reaction (binding interaction) between these two species. [Hint: there are two binding sites in MTMT for the Cu2+.]
If this binding were classified as an intermolecular interaction, which would it be?
[Answer 4]
4. The pKa of ethanol is 15.9 and the pKa of ethanethiol is 10.5. Draw structures for the two compounds and their conjugate bases (check chemspider.com if you're unsure about the structure).
What effect explains the difference in acidity between these two substances? [Hint: what is the difference between ethanol and ethanthiol? How will this difference affect the acidity/conjugate basicity?]
[Answer 5]
Try the problems from the MIT Open Course site Problem Set 1(#8); 2(#4); 5(#2b,c,d)
There are answers also.

When drawing the structure, first draw the methanethiol part, CH3SH, and then substitute the methylthio, CH3S-, for one of the hydrogens on CH3SH.
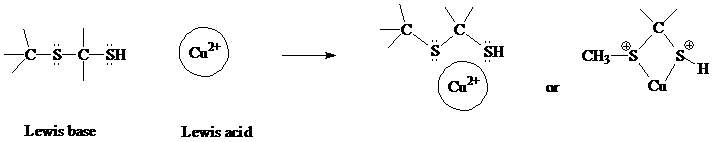
where each of the two sulfurs in the binding product should still have one non-bonding pair of electrons (unshown) and the other pair on each S is now the sigma bonding pair shown.
If this were classified as an intermolecular interaction instead of a Lewis acid-base reaction, the interaction would be between the sulfur (partial negative end of a dipolar bond) and the Cu2+ ion. So it would be an ion-dipole interaction.
Answer 5 Ignore the calculation of delta G and the resulting energy-level diagram.
