The Binding of Dodecaethylene glycol
(solvent) with the enzyme CLA-producing fatty acid isomerase
from P. acnes
Part 1: Choosing an enzyme-hetero complex to study
CLA-producing fatty acid isomerase (PDB ID: 2B9W), is an interesting enzyme found in anaerobic bacteria such as Propionibacterium acnes since it biologically produces CLA (Conjugated linoleic acid). This molecule has a been recognized as a cancer inhibitor, an antioxidant, a fat reducing agent and other effects that needs to be studied more (journal 2). It was not possible to find a complex that had CLA as a substrate but there was another molecule called Dodecaethylene glycol which seemed interesting and bound to this protein.
There were two main reasons why this “hetero-enzyme” complex was chosen. One of them is the fact that the CLA-producing fatty acid isomerase is an enzyme present in the bacteria Propionibacterium acnes which is an anaerobic bacterium that is associated as the primary bacterial cause of acne [5]. The second reason was the large change in energy the dodecaethylene glycol molecule showed from its bound state to its minimized state. This large change in energy urged the desire to study what possible use the enzyme made of this molecule. However after research was done to look for possible mechanisms in which the enzyme makes use of this substrate, no sources were found. An extremely helpful hint was given to me by Dr. Slayden who encouraged me to talk to Dr. Born about the connections between dodecaethylene glycol and the enzyme. Dr. Born then mentioned that this molecule like many polyethylene glycol (PEG’s) was just a solvent that is used specially for reactions involving enzymes such as lipase which work best with solvents with amphipathic since they increase the hydrophobicity of the solvent which make the interactions between the hydrophobic substrate with the fatty acid isomerase more likely. Secondly he mentioned that this solvent acts as a “cryoprotectant which lowers the freezing point of the solution. Being a solvent for an enzyme it was interesting to find what role do PEG’s in general have with the enzyme.
It turns out that since most enzymes are designed to operate under biological systems which are for the most part organic, linking the enzyme to an amphipathic polymers such as PEG’s, increase the solubility of the enzyme in organic solvents. Enzymes for the most part are insoluble in organic solvents and therefore would coagulate or aggregate preventing the effective binding of substrate [3]. PEG binding can therefore improve the solubility of the proteins in the organic solvents to facilitate their activity as it would be the case for the CLA-producing fatty acid isomerase of p. acne [3]. It is obvious that such enzyme would work under hydrophobic biological environments since its substrates are hydrophobic molecules. Some proteins however can do well without the aid of such solvent but probably the CLA-producing fatty acid isomerase from p. acne enzyme needs the dedecaethylene glycol solvent since it appears bound to the protein in every Hetero-protein complex found in the PSB site.
The nature of the organic solvents is important in maintaining the water content that would allow normal catalytic function [3]. Hydrophilic solvents for example would strip away essential hydration of water from the enzyme and causing distortion on the enzymes catalytic function [3]. For example in the case of Lipase which catalyzes the hydrolysis of tryacylglicerides would need water and a very hydrophilic solvent would drive most of the water molecules away from the enzyme thus preventing it from effectively catalyzing its function.
Part 2: Extraction of the Hetero-Compound and minimization
Enzymes can affect the structure
of a substrate they bind to either increase or decrease its energy through changes
in structure. The resulting change in structure will cause the substrate to
undergo various structural energy changes such as: Stretch,
|
Types of Energy parameters analyzed |
Native Energy of Substrate before minimization (kcal/mol) |
Energy of Substrate after minimization (kcal/mol) |
|
Stretch : |
12.4596 |
4.1069 |
|
Bend : |
49.8194 |
19.7942 |
|
Stretch-Bend: |
2.9811 |
2.1485 |
|
Torsion : |
17.5188 |
7.7902 |
|
Non-1,4 VDW : |
11.3201 |
-9.4576 |
|
1,4 VDW : |
51.6768 |
47.8603 |
|
Dipole/Dipole : |
7.4868 |
3.2048 |
|
Total: |
153.2626 |
75.4474 |
Table 1. Energy
Comparison before and after minimization of Dodecaethylene
Glycol
The first column in the table shows the Energy parameters measured by CHEM 3D. The second column shows the results of the energy values calculated by performing a “single point energy” which measures the energy state of the dodecaethylene glycol molecule as obtained from the protein. The second table in the column shows the results of the energy values when a geometric optimization or energy minimization process was done. This results essentially shows the energy values the dodecaethylene glycol would have if it were in a gaseous like environment.
Analysis of the energy parameters for Dodecaethylene:
Bond Stretching:
This energy term refers to the energy related in the stretching between any two atoms that are directly bonded to each other. The native stretch energy of the substrate before minimization was 12.4596 kcal/mol and after minimization it was 4.1096 kcal/mol as seen in the table above. Overall none of the bonds had any drastic change of bond length above 0.1Ao. This means that probably the molecule did not have any major stress to its bond lengths and therefore no drastic changes were seen.
Angle Bending:
This energy term refers to the energy related of the angle bending between atoms that are in a geminal position to each other, that is attached to the same atom and form an angle. Overall the largest changes in angle bending values were in the magnitude of 6-13 degrees. Although this measurements by themselves don’t look very big, when they are added to the overall energy of the molecule, their value is significant. The table also shows that the initial value for bending was 49.8194 kcal/mol and the energy minimized structure was 19.7942 kcal/mol which is one of the largest differences in energy compared to the other energy criteria. This makes sense since the molecule is twisted into a certain form to fit in the enzyme and therefore such bending of the angles causes the large difference in energy.
Torsion Energy:
The torsional energy represents the energy associated with the torsional angle rotations between atoms that are vicinal to each other. The value for the non-minimized structure came out to be 17.5188 kcal/mol compared to the value of the minimized structure which was 7.7902 kcal/mol. Although the values are separated by a somewhat large amount they are not the main source of energy change that is produced by the molecule although at first it was thought to be the case.
Non-VDW forces:
Theses forces deal with the steric hindrance between atoms due to large functional groups that are in proximity to one another. This compound does not have any large functional groups that might cause drastic changes in energy. The value of energy change however is somewhat large compared to the other energy values previously mentioned. In its original conformation the molecule has a energy of 11.3201 kcal/mol while it has an energy of -9.4576 kcal/mol after energy minimization was done.
Finally the energies associated with the 1,4 Van Der Waals energy don’t contribute to the energy difference very much as seen on the table. The same can be said for the Dipole/Dipole energies. The fact that the dipole/dipole energies did not contribute a large change can be explained through the fact that there are no largely polar groups along the molecule with the exception of the two hydroxyl groups at either end of the molecule. If they might have been somewhere in the middle of the molecule then probably this energy could have contributed more. The total energy for the molecule before the minimization was 153.2626 kcal/mol compared to the minimized energy of 75.4474 kcal/mol. As it can be seen the energy of the molecule went to almost half its initial value showing that the molecule had a lot of strain when bound to the protein. The picture below shows the resulting structure of the minimized energy state of Dodecaethylene.
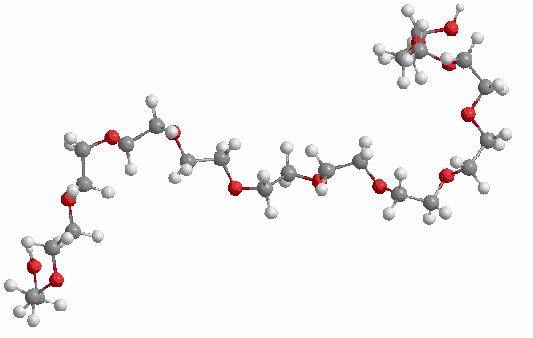
Figure 1. Structure of
the energy minimized Dodecaethylene glycol molecule
Part 3: Overlay of the Dodecaethylene
molecule in its Native state to its minimized state
The overlaying of the substrate in its native form (as found on the enzyme) and its minimized form is useful to visually observe the changes in structure that occurs in each state. As seen in the picture below the greatest change in structure is the bending of the molecule and this can easily be seen since the molecule is quite large. As seen in Table 1 the value for the change in the bending energies is the largest and contributes more to the total energy change. Although Stretching and Torsion are also some of the largest energy differences it is the Bending that can be seen easily. The molecule that is a little more coiled towards itself is the minimized structure, while the one that is more open is the native molecule.
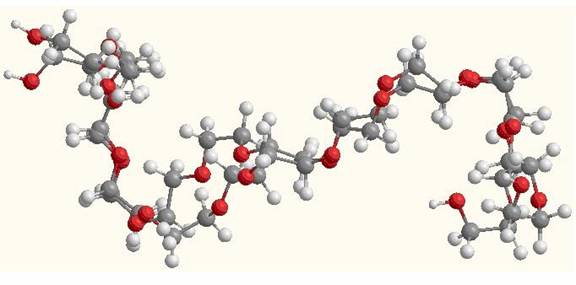
Figure 2: Overlay of native
and minimized Dodecaethylene glycol molecules.
Part 4: Interactions between the Hetero compound (Dodecaethylene glycol) and the enzyme
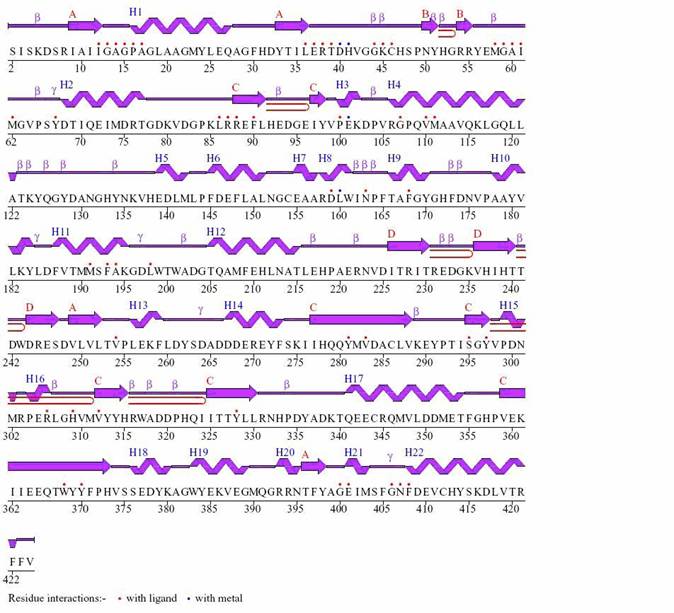
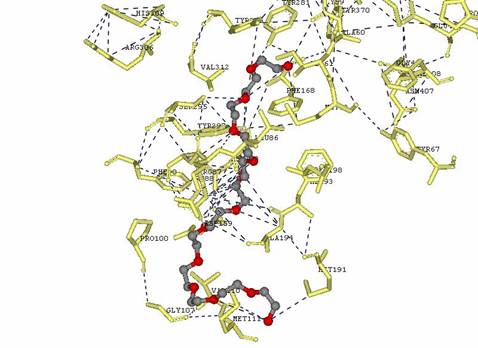
Figure 3: Wiring diagram for CLA-producing fatty acid isomerase from P. acnes with Dodecaethylene glycol
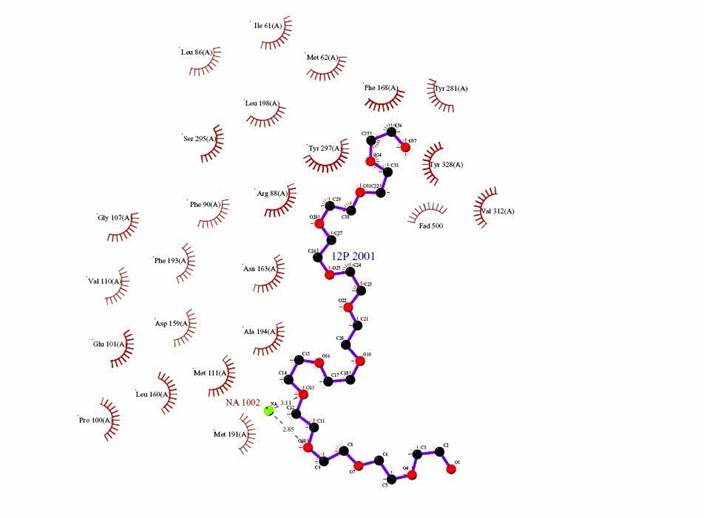
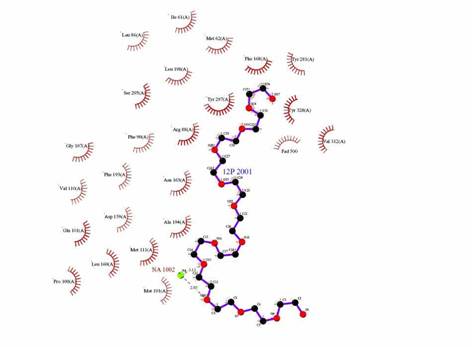
Figure 4: Ligplot of the interaction between Dodecaethylene glycol and the enzyme CLA producing fatty acid isomerase.
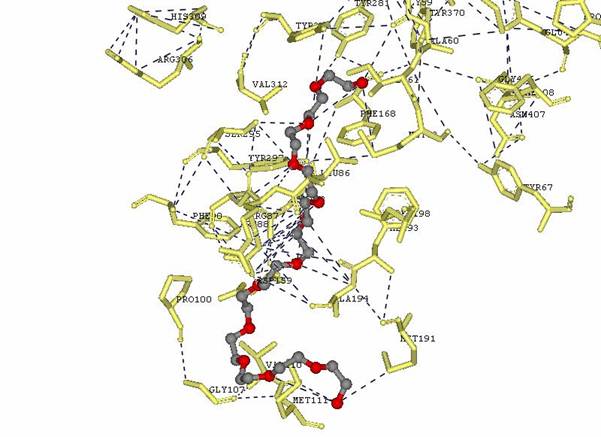
Figure 5: Image done in DS Viewer Pro to show residue amino acids with substrate interactions as shown in the ligplot.
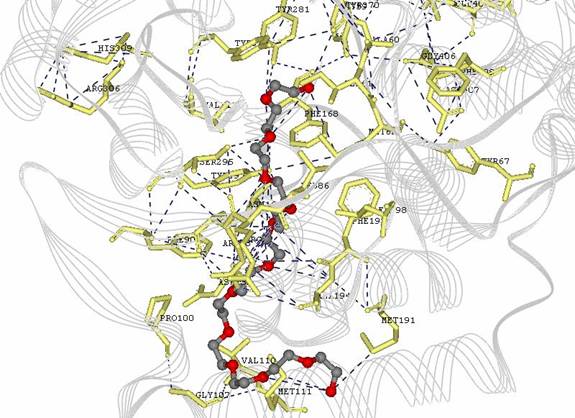
Figure 6: Image done in DS Viewer Pro to show amino acid residues with substrate interactions and overal protein in line model
|
|
|
Figure 6: Comparison of Ligplot from PDB Sum website and from DS Viewer Pro
The following table shows the main side groups that interact with the enzyme. The main interactions that are found are Hydrogen bond interaction due to the large amount of oxygen molecules present in the Hetero compound.
|
Table
2. Residues interacting with Dodecaethylene
glycol
Part V: Bibliography
1) Liavonchanka,
A., Hornung, E., Feussner,
2) Liu, J., Yong,
W., Deng, Y., Kallenbach,
N.R., Lu, M. ![]() Atomic
structure of a
Atomic
structure of a
tryptophan-zipper pentamer.
![]() Proc.Natl.Acad.Sci.USA
Proc.Natl.Acad.Sci.USA ![]() v101
v101 ![]() pp.16156-16161 ,
2004.
pp.16156-16161 ,
2004.
PDB ID: 1T8Z. (Another enzyme that binds dodecaehtylene
glycol, the original article is not in the GMU database). http://www.pdb.org/.
3) Zacchigna, M.; Luca, G.; Lassiani, L.; Varnavas, A.; Boccu,
4) Lee, S.; Hong, W.; Oh, K. Biotechnol. Prog. 19 2003. Bioconversion of Linoleic Acid into Conjugated Linoleic
Acid by Immobilized Lactobacillus reuteri.
Pp 1081-1084. Retrieved from ACS database on May 9, 2006.
5) Tortora, G.; Funke, B.; Case, C. “Microbiology” an Introduction. 7th Ed.; Benjamin Cummings, 2002; pp 325.
Acknowledgements to:
Dr. Suzanne W. Slayden for referring me to Dr. Born for help and to Dr. Timothy S. Born for helping me understand the function of solvents when studying proteins.