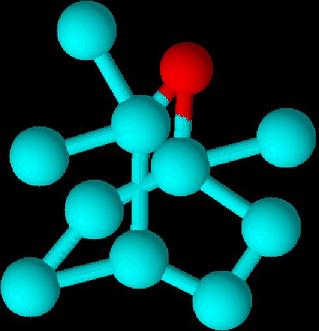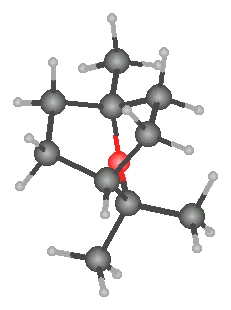
1,8-Cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane) is a bicyclic ether and a monoterpene.[14][15] It is synthesized by plants to suppress the growth of the same or other species of plants that are nearby.[15] 1,8-Cineole is a chemically unreactive molecule since it has no reactive C-H bonds.[14] Not much is known about the chemistry of this compound except for the cleavage of the ether bridge which produces p-menthane derivatives.14 Most of the derivatives of 1,8-Cineole are made by using a-terpineol or pinol.[15][16]
The 1,8-cineole synthase (CS) is used to catalyze to formation of 1,8-cineole in plants. CS converts (R)-linaloyl PP into the (R)-LDP intermediate by allylic rearrangement. Then (R)-LDP is converted to (R)-a-terpinyl cation by an anti,endo SN’ cyclization. After the cyclization, a syn addition of water occurs at C7 to produce the (R)-a-terpinyl hydronium ion. Then a proton in the acid solution is attacked by the cyclohexene double bond which causes the cyclication and of the C=C double bond and the hydroxyl group to produce 1,8-cineole.[15]