Ethyl Cellulose
Polymers are the giants of organic chemistry. They are chains of molecules linked together in myriad combination. Some of these polymers occur naturally and can be found in nature such as polysaccharides and proteins. Others are created artificially in labs like poly vinyl chloride or polyethylene. One of the natural occurring polymers is cellulose; it is in the polysaccharide family. A polysaccharide is a polymer in which the monomer, or parent molecule, is a sugar (1). The monomer for cellulose is glucose, a very common sugar in most plants and animals. In fact cellulose is one of the most abundant natural polymers in the world it is what plant cell walls are made of, and ninety-percent of cotton and fifty-percent of wood is comprised of cellulose (4). Cellulose can in turn have many different variations such as ethyl cellulose. Ethyl Cellulose is a natural occurring polymer too. It is found in wood and can be extracted from wood pulp.
The cellulose ether industry began in 1912 with the work of Lilienfeld. Cellulose and its derivatives, however, have been around since plants have so an exact time on their discovery is hard to pinpoint (6).
"Ethyl Cellulose is a cellulose ether in which ethyl groups replace hydrogen in the hydroxyl groups of glucose residues."(3) In other words it is made by the reaction of alkali cellulose with ethyl chloride (4). The alkylization step makes use of 50% or greater solutions of NaOH. In a nickel-lined pressure vessel the ethylation process takes place over a period of ten to twenty hours at the temperature of 90-150º C. There are two methods of extraction: batch making or semi-continuous, which varies the collection of the product and when and how it is washed (6).
The monomers of Ethyl Cellulose are below one is cellulose (1).
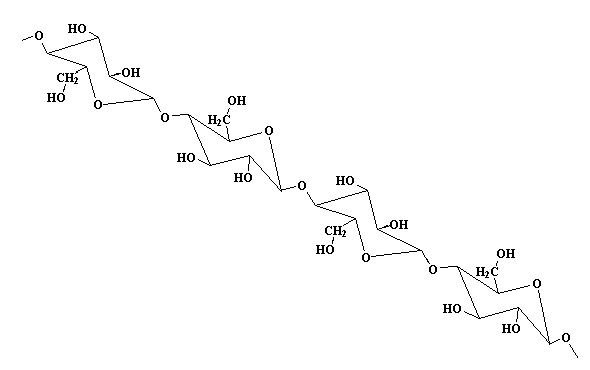
The other monomer is ethyl chloride. CH3CH2Cl
Cellulose has a monomer called glucose (1):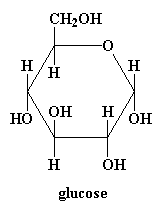
Ethyl cellulose is an off white granular powder that is hazardous to humans so contact and inhalation should be avoided (5). Ethyl cellulose is known for its toughness and its strength (6). It is a thermal setting plastic, which means once it is made into a plastic solid it is virtually indestructible. It can be destroyed, however, if it is mixed with a combustible solvent and burned in a chemical incinerator; the incinerator should have an afterburner and scrubber though. The powder form will decompose into carbon dioxide and carbon monoxide and both can be hazardous to humans (5).
"Ethyl Cellulose products are used as inhibiting materials for rocket-propellant grains."(2) It is also used to make electrical appliance parts, fire extinguisher components, flashlight cases, and pill binders in the pharmaceutical world (3). It is also used to make lacquers and thermo plastics for example the protective coating on bowling ball pins. It is also found in printing inks, hot melt adhesives, football helmets, and tool handles (6).
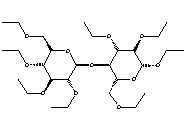
Here is the final product of the reaction between ethyl chloride and alkali cellulose also known as ethyl cellulose (7).
Sources
1. http://www.psrc.usm.edu/macrog/cell.htm
2. http://www.islandgroup.com
3. http://me.mit.edu/2.01/Taxonomy/Characteristics/Cellulose.html
4. http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2000/Cellulose/main%20page.htm
5. http://www.camd.lsu.edu/msds/e/ethyl_cellulose.htm
6. Encyclopedia of Industrial Chemical Analysis Volume 9. Edited by Foster Dee Snell and Leslie S. Ettre: Interscience Publishers © 1970.
7. http://www.chemACX.com



