|
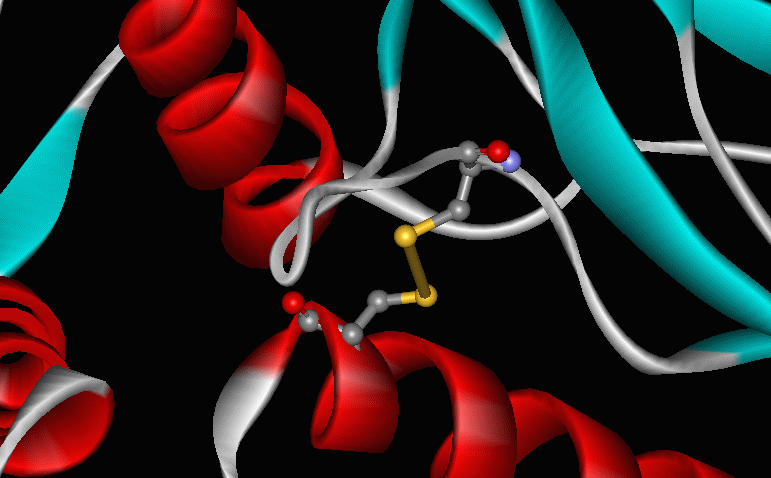
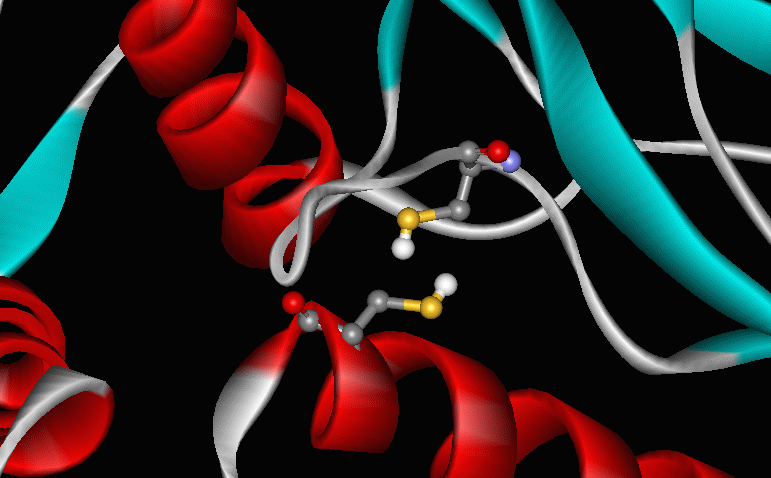
An intramolecular disulfide bond serves as a reversible switch to turn on or off a protein that helps protect bacterial cells from damaging oxidation reactions. The protein, OxyR, responds specifically to elevated levels of hydrogen peroxide, which could damage DNA, lipid membranes, or proteins. Researchers have shown that high levels of H2O2 trigger formation of a disulfide bond between two specific cysteine residues of OxyR. The S-S bond disappears when the protein is reduced. |
|
R-SH + R'-SH ---> R-S-S-R' [oxidation]
|
|
R-S-S-R' ---> R-SH + R'-SH [reduction] A reaction that usually inactivates cytoplasmic proteins -- the oxidation of cysteines to form intramolecular disulfides -- has been exploited as an on-off switch. |